Stage IV breast cancer, which has a high risk of invasion, often develops into metastases in distant organs, especially in the lung, and this could threaten the lives of women. Thus, the development of more advanced therapeutics that can efficiently target metastatic foci is crucial.
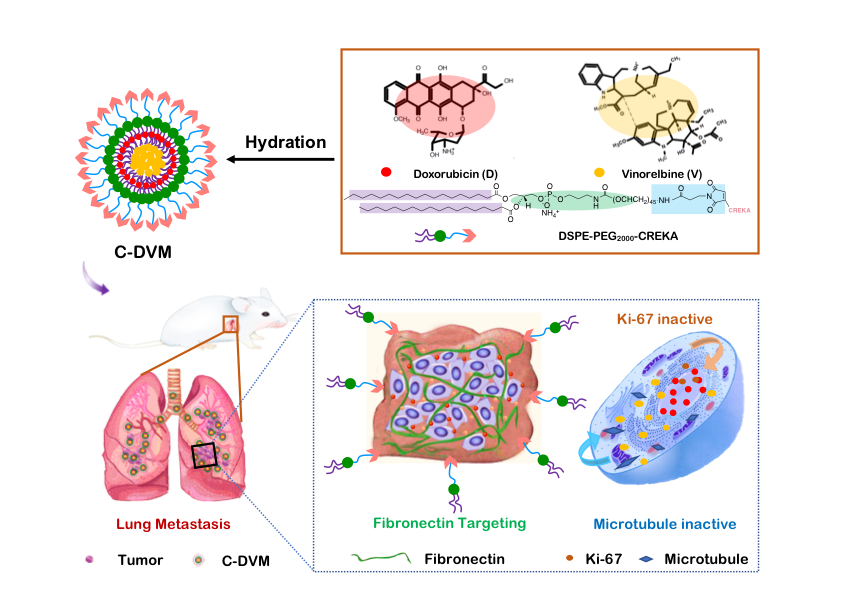
In this study, we built an dual-acting therapeutic strategy using micelles with high stability functionalized with fibronectin-targeting CREKA peptides encapsulating two slightly soluble chemotherapy agents in water, doxorubicin (D) and vinorelbine (V), which we termed C-DVM. We found that small C-DVM micelles could efficiently codeliver drugs into 4T1 cells and disrupt microtubule structures. C-DVM also exhibited a powerful ability to eradicate and inhibit invasion of 4T1 cells. Moreover, an in vivo pharmacokinetics study showed that C-DVM increased the drug circulation half-life and led to increased enrichment of drugs in lung metastatic foci after 24 h. Moreover, dual-acting C-DVM treatment led to 90% inhibition of metastatic foci development and reduced invasion of metastases. C-DVM could potentially be used as a targeted treatment for metastasis and represents a new approach with higher therapeutic efficacy than conventional chemotherapy for stage IV breast cancer that could be used in the future.